Evergreen Therapeutics, Inc. Announced to Develop EG-009 for the Treatment of Moderate to Severe COVID-19
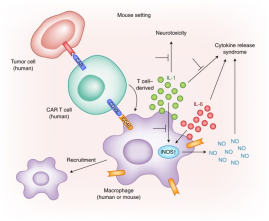
In April 2020, Evergreen Therapeutics, Inc. announced the development of a new pipeline, EG-009, to treat the Cytokine Release Syndrome (CRS) caused by the Novel Coronavirus pneumonia. Epidemiologists predict that the world will remain in a pandemic state until June 2021. The virus continues to spread at a slow rate, and intermittent lockdowns are the new normal. It is estimated that 250 million people worldwide will be infected and 1.75 million will die by June 2021.CRS is one of the leading causes of death in COVID-19 patients. CRS is a complex inflammatory response. During the occurrence of CRS, various cytokines such as TNF-, IL-1, IL-6, IL-12, IFN-, IFN-, McP-1, and IL-8 are produced in large quantities in body fluids. CRS can cause acute respiratory distress syndrome and mu