
Dr.Charles Lee, CMO of Evergreen
Dr. Li Changqing, Chief Medical Officer (“CMO”) of Evergreen Therapeutics (“Evergreen” or the “Company”) shared his excitement on the very intuitive demonstration of the efficacy of the EG-007 combination: “Simply comparing the size of tumor residual in the same animal trial, the average tumor weight after dual-drug treatment was approximately 730 mg, while after our three-drug regimen it was just over 300 mg.”
EG-007 was the first drug candidate Dr. Li led after joining Evergreen. In mid-July this year, Dr. Li assumed the position of Evergreen CMO. Subsequently in August, after two pre-IND meetings, the FDA approved the Phase III pivotal clinical trial protocol of EG-007 in combination with targeted drugs and anti-PD-1 antibody analogs for the treatment of advanced endometrial cancer. Notably, the "cola combination" of Pembrolizumab (K Drug) and Lenvatinib, regarded as the "dark horse" in cancer treatment, just recently received full FDA approval for the treatment of patients with advanced non-microsatellite highly unstable (MSI-H) or mismatch repair defect (dMMR) endometrial cancer.
Despite the cola combination not having been approved in China, the Chinese Clinical Oncology Association has paid attention, in particular as it relates to the treatment guidelines for endometrial cancer. According to Dr. Li, a Phase III key clinical trial is underway, but the individual drugs in the combination are marketed separately in China, so in practice, there is no threshold for combination use. This means that the cola combination has advanced the treatment of endometrial cancer to new heights to become the worldwide "standard of care" in this field. Therefore, EG-007 will directly challenge this new treatment standard in Phase III clinical trials.
Evergreen has designed a superiority test for EG-007 that is not only statistically significant but also clinically meaningful. Dr. Li explained: “People may have a misconception, that when they first hear about a drug candidate going directly into Phase III, they think it is very impressive; then they look at the product, and see that it is an 'old drug’, so think there is no bright spot. Actually not all old (or existing) drugs can directly entered Phase III studies. The FDA pays more attention to the clinical value behind a project.”
Before entering the industry, Dr. Li served as a senior medical review officer at the FDA, and he was the first mainland Chinese student to join the FDA clinic review committee as a physician. Familiarity with FDA regulations, coupled with years of review experience, makes Dr. Li more capable and ready in leading the advancement of the EG-007 project. In addition to Dr. Li, other Evergreen executives, including Chairman Du Tao, CEO Du Xin, and Pharmacology Director Hu Tao who is specifically involved in the EG-007 project, all had experience in the FDA. It is these invaluable experiences that distinguishes Evergreen from most other biotech companies in China.
In expressing his expectations for the EG-007 project, Dr. Li commented: “We are exploring a new methodology, a new path that can increase clinical value.” EG-007's initial success in endometrial cancer is just a small step for Evergreen. Once the "existing drug repurpose" model is established, Evergreen expects to rapidly replicate it in other indications and pipelines to drive results to market.
01
"Old" or "new" drug, the key is to improving clinical value
In 2018, the new "18A" policy for Hong Kong stock listings came into effect, and ushered in a wave of IPOs for unprofitable biotech companies in China. Subsequently, the launch of the Science and Technology Innovation Board further contributed to the trend of local innovation in biopharmaceuticals, and topics such as “first in class” and “best in class” frequently occupied the focus of industry discussions. The problem however is that “first in class” is a concept that has not fully reached industry consensus. A large number of projects boasting “first in class” are piling up in a few niche markets, creating a bubble-like situation in the domestic innovation environment. In July this year, China's Center for Drug Evaluation (CDE) released the "Clinical Value-Oriented Clinical Development Guidelines for Antineoplastic Drugs" for consultation, with the goal of correcting the innovation chaos.
“From our perspective, first in class and best in class are of course great, if they can be achieved. However, these concepts are only superficial. Whether a drug can be approved, or whether it should be given the appropriate support, depends on whether it can bring new clinical value, that is, solve an unmet clinical need,” Dr. Li responded. The FDA, for example, has opened special lanes for priority review, fast track, breakthrough therapy and accelerated approval, the purpose of which is to support drugs that have the potential to improve patient benefits at different stages, from development to marketing. The cola combination was granted “Breakthrough Therapy” designation by the FDA in 2018 due to favorable interim results from the Study 111/KEYNOTE-146 on an endometrial cancer cohort. Looking at the mechanism of action of the cola combination, the K drug primarily blocks the PD-1 immune escape pathway, while Lenvatinib inhibits the growth and differentiation of tumor cells and TAM by competitively inhibiting the binding of VEGF and the corresponding receptors - whichever one it is, and both have been previously researched in a number of well-established studies.
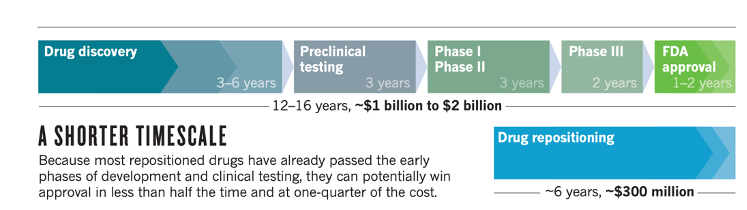
Development time, cost and patent protection for old drugs
Dr. Li commented: “If using the strict criteria that designate drugs which have been marketed and approved as old drugs, then even the PD-1 monoclonal antibody, which has been hailed as an anti-cancer miracle drug, is an old drug. However, this does not affect its subsequent development; as long as the indications and combinations bring benefit, they will be welcomed by the drug regulatory authorities.” Not only are international pharmaceutical companies increasingly focusing on the strategy of “old drugs, new use,” but research institutions such as the NIH also see it as a strategic area. In the past decade, the NIH has repeatedly pointed out in different ways that it wants to help speed up drug development and cut costs by testing approved drugs for different diseases.
Comparing development time, costs and patent protection between a new drug and old drug repurposed, the latter poses many attractive opportunities. A new drug takes roughly 10 years and costs $1 billion, with a 10% success rate; the success rate is even lower in the anti-tumor field, at less than 4%. An old drug repurposed requires about 6 years and costs $300 million, and with much higher success rates at 25%. Improving clinical value is not limited to improvements in efficacy, but also depends to a large extent on patient burden; new use of old drugs is raising success rates and lowering R&D costs, contributing to a win-win situation for developing "good drugs at low prices”. Furthermore, new use of old drugs is subject to very tight patent protection. Whether in China, Europe, or the United States, if an old drug is researched and a new use is found, a “new-use patent" can be applied. The new-use patent is a type of invention patent with strong and wide scope of protection. Therefore the new use of old drugs is a new trend in the international pharmaceutical industry.
“It is our belief to make the seemingly ordinary process of new use for old drugs the ultimate goal, remarked Dr. Li, where ‘ultimate’ is similar to making beads for a crown on the head of a giant, and the EG-007 project is the realization of this belief.” Since only one Phase III clinical trial was required, and the combination consisted of approved old drugs, eliminating the need for additional proof of single-drug effectiveness, Evergreen saved 60% in cost and time. The core of EG-007’s three-drug combination revolves around improving the internal tumor microenvironment (TME), turning "cold tumors" into "hot tumors" with the synergy of different mechanisms, and increasing T-cell infiltration in tissues to enhance the tumor-killing effect. This is the key contribution to the combination and mechanism of action. Based on data from Dr. Li, Evergreen's triple drug combination inhibited 86% of tumors in animal models, compared to 75% for the "cola combination" and 69% for monotherapy (Anti-PD1 antibody).
02
EG-007 overcomes "three mountains" compared to the "cola combination"
Dr. Li describes the EG-007 project as: “Rapid entry into phase III clinical stage, selection of the best combination, "head-to-head" drug value positioning.” The EG-007 project has been promoted according to the direct-to-market model from the beginning. This strategy is not an attempt to take advantage of the rapidly diminishing window of domestic biopharmaceutical innovation and capital market windfall. On the contrary, EG-007 is already Evergreen's fourth drug candidate to enter clinical stage. EG-007 was initially filed as a small-scale Phase II clinical application, and after certain clinical endpoints were met, would be used as part of Phase III clinical profile to continue development studies.
Dr. Li explained: “We developed a 'wick strategy.’ Just like how you use a fuse to light a candle, we designed a small part of the clinical program, similar to lighting a wick, so that only 20 or 30 patients were needed for Phase II clinical trials. After that, we moved into Phase III clinic trials at a scale of five to six hundred patients. Considering the therapeutic needs of endometrial cancer and the response rate level of existing drugs, during formal communications, the FDA proposed another option for EG-007 to skip Phase II and enter Phase III directly, which was the reason for a rare second pre-IND meeting. The final ability to complete smooth communication with the FDA also depended on the accumulation of regulatory background of the team.”
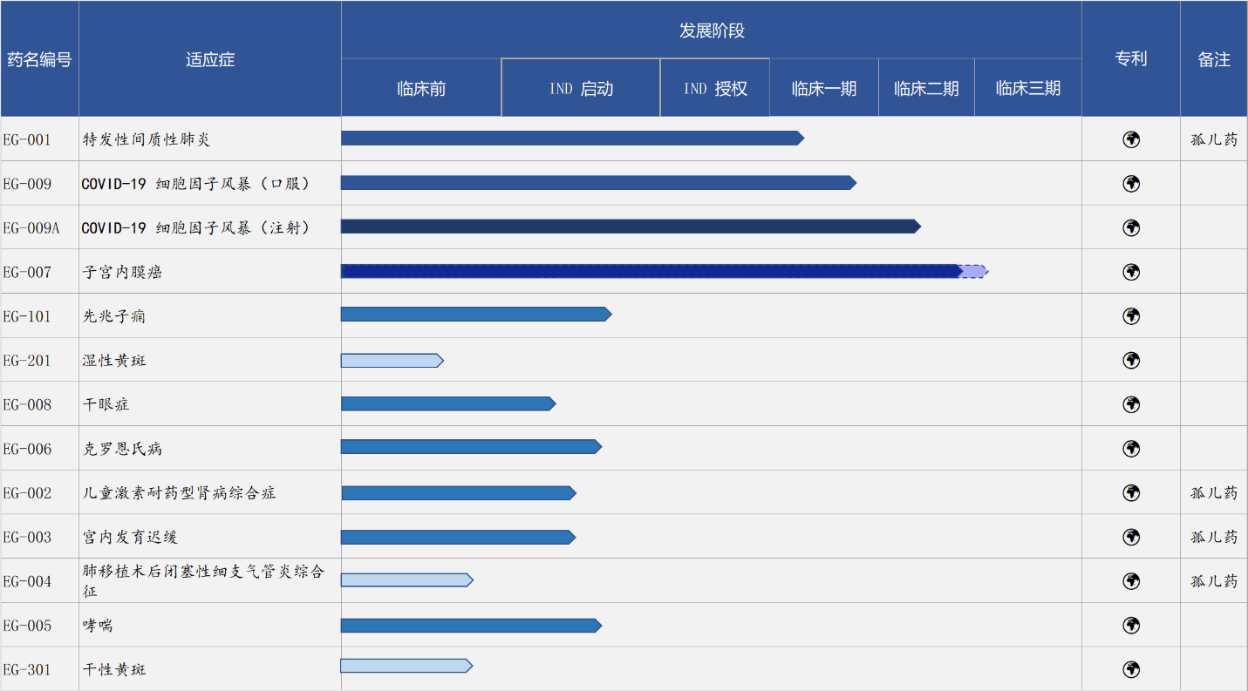
Pipeline of Evergreen
“The judgment of where the FDA's policy is flexible or less flexible is very important. Endometrial cancer is among the most common types of cancer in the uterus and the sixth most common cancer in women worldwide. Data show that there will be more than 417,000 new diagnoses and 97,000 deaths worldwide in 2020. The emergence of the cola combination provided more hope for endometrial cancer patients and allowed Evergreen to see the direction of the benchmark. The EG-007 project was developed based on the tumor microenvironment. When selecting the combination, we conducted a comprehensive analysis of existing related therapies and settled on the cola combination. For tumor immune combination therapy, it should be considered one of the best, and 14 indications have been laid out, including endometrial cancer, which have been explored with EG-007,” Dr. Li added.
Regarding the project idea, Dr. Li explained: “There are still very few new drugs approved in this field, and there is a lot of room to improve efficacy. We want to stand on the shoulders of giants and do better. Compared with the chemotherapy group, the objective remission rate (ORR) of patients with endometrial cancer treated with the cola combination doubled, but it was still only 30 percent. The question was whether Evergreen's EG-007 three-drug combination should start with the 70% of patients not covered by the cola combination, or be a direct challenge to the cola combination. It comes down to the clinical positioning of the drug, whether it should be a first-line, second-line, or third-line treatment.”
According to Dr. Li, and considering previous studies, the EG-007 program can directly compete with the existing cola combination as the preferred treatment option. “As we all know, it is a routine approach in many clinical trials to provide medical treatment for a previous treatment failure. After all, the mechanism is not greatly changed, and adding new drugs to improve the tumor microenvironment may improve the efficacy of the combination. However, if the result of combination treatment is still not successful, the situation will become more complicated; it may be due to insufficient improvement of the microenvironment, or the patient may have impedance problems, or other unknown factors. On the other hand, from the perspective of animal models, it is difficult to find animals that have failed treatment and add new drug candidates for MOA validation. So although we feel that positioning EG-007 as a first-line treatment and a direct challenge to existing cola combination therapy carry risks, it is still a more favorable option overall,” Dr. Li concluded.
Based on Evergreen's plans, the EG-007 program will conduct Phase III clinical trials at more than 100 clinical trial sites, covering more than 10 countries and regions. After obtaining an IND in the United States, clinical filings for major drug markets such as Europe, Japan, and China are gradually on the agenda. “We are communicating with several CROs to start cooperation for this clinical advancement. The optimistic estimate is to strive to complete a pivotal Phase III trial within 3 years and submit new drug applications globally within that time frame,” commented Dr. Li. By that time, Evergreen’s methodology of moving from "first in disease”, to "first in treatment choice”, to "first in patient choice" will be backed by solid case studies.



